Freezing Point of Water: The Ultimate Guide

<!DOCTYPE html>
Understanding the freezing point of water is essential for various applications, from scientific experiments to everyday cooking. At 0°C (32°F), water transitions from a liquid to a solid state, forming ice. This process is influenced by factors like pressure, impurities, and the presence of dissolved substances. Whether you’re a student, a chef, or simply curious, this guide will provide valuable insights into the freezing point of water, its significance, and practical applications.
What is the Freezing Point of Water?

The freezing point of water is the temperature at which water changes from a liquid to a solid (ice). Under standard atmospheric pressure, this occurs at 0°C (32°F). However, this can vary based on external conditions. For instance, saltwater has a lower freezing point due to the presence of dissolved salts, a phenomenon known as freezing point depression.
💡 Note: Pure water freezes at 0°C, but impurities or dissolved substances can alter this temperature.
Factors Affecting the Freezing Point of Water
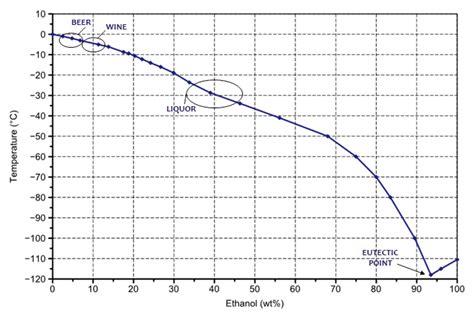
Several factors influence the freezing point of water:
- Pressure: Higher pressure can slightly lower the freezing point.
- Impurities: Substances like salt or sugar lower the freezing point.
- Container Material: Some materials can affect how quickly water freezes.
Understanding these factors is crucial for applications like food preservation, weather forecasting, and chemical experiments.
Practical Applications of Water’s Freezing Point

The freezing point of water plays a vital role in various fields:
| Application | Description |
|---|---|
| Food Preservation | Freezing is used to preserve food by slowing bacterial growth. |
| Weather Monitoring | Understanding freezing points helps predict frost and ice formation. |
| Chemical Reactions | Many reactions are temperature-dependent, relying on precise freezing points. |

For those looking to buy laboratory equipment or kitchen appliances, knowing the freezing point ensures optimal performance.
How to Measure the Freezing Point of Water
Measuring the freezing point of water can be done using simple tools:
- Use a thermometer to monitor temperature.
- Place water in a container and gradually lower the temperature.
- Observe the point at which water begins to solidify.
For precise measurements, consider using a digital thermometer or specialized equipment like a cryoscope.
Checklist for Experimenting with Water’s Freezing Point

- Ensure water is pure for accurate results.
- Use a calibrated thermometer for precise measurements.
- Control external factors like pressure and container material.
- Record observations for analysis.
From scientific experiments to culinary techniques, the freezing point of water is a fundamental concept with wide-ranging applications. By understanding the factors that influence it and how to measure it accurately, you can harness its potential in various fields. Whether you’re preserving food, predicting weather, or conducting experiments, this guide has provided the essential knowledge you need.
What is the freezing point of pure water?
+The freezing point of pure water is 0°C (32°F).
How does salt affect the freezing point of water?
+Salt lowers the freezing point of water through a process called freezing point depression.
Why is the freezing point of water important in food preservation?
+Freezing slows bacterial growth and enzymatic activity, extending the shelf life of food.


